
Public Comments Processing
Attn: FWS-HQ-ES-2021-0033
U.S. Fish and Wildlife Service
MS: PRB/3W
5275 Leesburg Pike
Falls Church, VA 22041-3803
Re: Proposed Revisions to Regulations Regarding Experimental Populations Under the Endangered Species Act, Docket No. FWS-HQ-ES-2021-0033
The Southern Environmental Law Center submits the following comments on behalf of a broad coalition of 24 conservation groups working in the Southeast, in support of the U.S. Fish and Wildlife Service’s (“FWS” or “Service”) proposal to revise the regulations governing the reintroduction of experimental populations of threatened and endangered species under section 10(j) of the Endangered Species Act (“ESA”), 16 U.S.C. § 1539(j). Proposed Rule, Designation of Experimental Populations, 87 Fed. Reg. 34,625 (June 7, 2022) (to be codified at 50 C.F.R. Part 17).
The use of experimental populations as a means to reintroduce species into their historic range is a well-established conservation tool and has been widely successful in helping to recover threatened and endangered species. As climate change continues to alter habitats and force species and ecosystems to migrate into new areas, the ability to introduce species into new areas beyond their historic range could serve as an essential tool for recovering imperiled species. Current levels of habitat loss already hamper species’ abilities to adapt to climate change and further diminishes the availability of suitable habitat.
The Service’s proposal to remove the “historic range” limitation on experimental populations will provide the agency with needed flexibility to take effective conservation action in the face of such dynamic and unpredictable circumstances. We support FWS’s recognition of the impacts of climate change on species and its forward-thinking proposal to protect imperiled species in the face of these threats. As detailed in these comments, such action is necessary to help conserve the Southeast’s rich biodiversity in the face of accelerating threats and will further the ESA’s conservation purposes.
I. THE SOUTHEAST’S IMPERILED BIODIVERSITY
The climate and geography of the Southeastern United States have enabled the region to harbor high levels of biodiversity for millions of years,1See, e.g., Reed F. Noss et al., How Global Biodiversity Hotspots May Go Unrecognized: Lessons from the North American Coastal Plain, DIVERSITY & DISTRIBUTIONS (2015), provided as Attachment 1. recognized as the 36th Global Biodiversity Hotspot in 2016.2Reed F. Noss, Announcing the World’s 36th Biodiversity Hotspot: The North American Coastal Plain, CRITICAL ECOSYSTEM PARTNERSHIP FUND (Feb. 18, 2016), https://www.cepf net/stories/announcing-worlds-36th- biodiversity-hotspot-north-american-coastal-plain. To qualify for that title, an area must have over 1,500 endemic plant species and must have lost at least 70 percent of its natural habitat; the Southeast exceeds these requirements, hosting over 1,800 endemic plant species and having 85.5 percent of its natural habitat “highly altered or converted to anthropogenic land cover.”3Id. The rivers and streams of the Southeast in particular support an astounding level of biodiversity relative to the rest of the United States, hosting 38 percent of the entire country’s freshwater fish species, 43 percent of its snails, 60 percent of its mussels, and 52 percent of its turtles.4Charles Lydeard & Richard L. Mayden, A Diverse and Endangered Aquatic Ecosystem of the Southeast United States, CONSERVATION BIOLOGY (Aug. 1995), provided as Attachment 2.
Unfortunately, Southeastern ecosystems are as imperiled as they are diverse. Because of such dramatic habitat loss, many species in the region have suffered devastating population declines. The ESA plays an important role in protecting imperiled species from humanmade threats. Across the Southeast, there are currently 258 species protected by the ESA as endangered (177), threatened (78), or experimental populations (32).5To compile these numbers, SELC reviewed FWS’s Environmental Conservation Online System, NMFS’s Species Directory, Federal Register notices, and the Code of Federal Regulations. A full list of listed Southeastern species is available upon request. More than half of these inhabit freshwater ecosystems, including 101 species of mussels, snails, and crayfish, as well as 36 fish species.6Id.
A. Habitat Loss Threatens Imperiled Southeastern Species
Despite efforts to prevent extinction, biodiversity loss remains a significant and rapidly increasing problem in the Southeast, across the United States, and abroad. The Southeast’s habitats currently face many threats from human activities, with habitat degradation and destruction as the leading causes of extinction.7See, e.g., Stuart L. Pimm et al., The biodiversity of species and their rates of extinction, distribution, and protection, SCI. (May 30, 2014), provided as Attachment 3; David S. Wilcove et al., Quantifying threats to imperiled species in the United States: Assessing the relative importance of habitat destruction, alien species, pollution, overexploitation, and disease, BIOSCIENCE (Aug. 1998), provided as Attachment 4.
As one of the fastest-growing areas of the country,8See U.S. Census Bureau, Press Release, Southern and Western Regions Experienced Rapid Growth This Decade, U.S. DEP’T OF COMMERCE (May 21, 2020), https://www.census.gov/newsroom/press-releases/2020/south-west- fastest-growing.html. the Southeast currently experiences many forms of habitat degradation—including from development, logging, agriculture, pollution, poor land management, and introduction of invasive species, among others. As cities expand, urban sprawl fragments and destroys previously intact natural habitats, introducing a host of threats to wildlife.9Adam J. Terando et al., The Southern Megalopolis: Using the Past to Predict the Future of Urban Sprawl in the Southeast U.S., PLOS ONE (July 23, 2014), provided as Attachment 5. Habitat fragmentation harms species and their habitats by diminishing water quality, interrupting predator-prey relationships, decreasing the availability of foraging habitat, and hindering resilience from disturbance.10Id. See also, e.g., Nick M. Haddad et al., Habitat fragmentation and its lasting impact on Earth’s ecosystems, SCI. ADVANCES (Mar. 20, 2015), provided as Attachment 6; Maxwell C. Wilson et al., Habitat fragmentation and biodiversity conservation: Key findings and future challenges, LANDSCAPE ECOLOGY (Nov. 20, 2015); Lenore Fahrig, Effects of habitat fragmentation on biodiversity, ANNUAL REVIEW OF ECOLOGY, EVOLUTION, & SYSTEMATICS (2003); Ilkka Hanski, Habitat loss, the dynamics of biodiversity, and a perspective on conservation, AMBIO (Mar. 18, 2011). Densely developed areas may also facilitate the expansion of invasive species.11Sean B. Menke et al., Urban areas may serve as habitat and corridors for dry-adapted, heat tolerant species; An example from ants, URBAN ECOSYSTEMS (Sept. 9, 2010).
B. Climate Change Will Exacerbate Threats to Imperiled Southeastern Species
To further compound these threats, climate change is predicted to significantly transform habitats throughout the Southeast in the near future, introducing additional harm to the already imperiled species and habitats in the region.12Lynne Carter et al., Southeast, in IMPACTS, RISKS, & ADAPTATION IN THE UNITED STATES: FOURTH NATIONAL CLIMATE ASSESSMENT, VOL. II, 743-808 (David Reidmiller et al. eds., 2018), provided as Attachment 7; Douglas Lipton et al., Ecosystems, Ecosystem Services, and Biodiversity, in IMPACTS, RISKS, & ADAPTATION IN THE UNITED STATES: FOURTH NATIONAL CLIMATE ASSESSMENT, VOL. II, 268-321 (David Reidmiller et al. eds., 2018), provided as Attachment 8. The Intergovernmental Panel on Climate Change reports that human activities are estimated to have caused approximately 1.0°C (1.8°F) of global warming above pre-industrial levels, and global warming is likely to reach 1.5°C (2.7°F) between 2030 and 2052 if temperatures continue to increase at the current rate.13Intergovernmental Panel on Climate Change (IPCC), 2018: Summary for Policymakers, in SPECIAL REPORT: GLOBAL WARMING OF 1.5°C (Valérie Masson-Delmotte et al. eds., 2018), https://www.ipcc.ch/sr15/chapter/spm/. Approximately 5 percent of global terrestrial land area may be expected to completely change ecosystem types (e.g., from temperate forest to arid savanna) at this level of warming.14Id. at 10.
Climate change will lead to habitat degradation and/or loss in the Southeast in myriad ways, including: higher temperatures, extreme precipitation, increased drought, more frequent and intense wildfires, rising sea levels, increased flooding, higher invasive species prevalence, shifting ocean currents, and increased storm frequency and intensity.15Lynne Carter et al., supra note 12. As a result, it is likely that the Southeast will see large species range shifts in the coming decades, but ongoing development and urban sprawl in the Southeast will almost certainly hamper the ability of species to move in response to these threats.16Lee Hannah, Climate change, connectivity, and conservation success, CONSERVATION BIOLOGY (Dec. 2011), provided as Attachment 9. Biodiversity loss can and should be minimized by climate-smart policies that protect potential future habitats for and facilitate the movement of imperiled species.17See, e.g., Lynne Carter et al., supra note 12; Emma P. Gómez-Ruiz & Thomas E. Lacher, Jr., Climate change, range shifts, and the disruption of a pollinator-plant complex, SCI. REPORTS (Oct. 1, 2019).
II. THE ROLE OF EXPERIMENTAL POPULATIONS UNDER THE ENDANGERED SPECIES ACT
The ultimate goal of the ESA is to achieve recovery of threatened and endangered species through conservation actions, where “conservation” is defined as “the use of all methods and procedures which are necessary to bring any endangered species or threatened species to the point at which the measures provided pursuant to this chapter are no longer necessary.” 16 U.S.C. § 1532(3). The conservation and recovery purposes permeate the entire statute. See, e.g., id. § 1531(c)(1) (“It is further declared to be the policy of Congress that all Federal departments and agencies shall seek to conserve endangered species and threatened species and shall utilize their authorities in furtherance of the purposes of this chapter.”); id. § 1533(d) (requiring the Service to “provide for the conservation” of listed species); id. § 1536(a)(2) (requiring federal agencies to ensure their actions are “not likely to jeopardize the continued existence of any endangered species or threatened species”). As summarized by the Supreme Court, “[t]he plain intent of Congress in enacting this statute was to halt and reverse the trend toward species extinction, whatever the cost.” Tenn. Valley Auth. v. Hill, 437 U.S. 153, 184 (1978).
A. The ESA 10(j) Legal Framework
Section 10(j) of the ESA, authorizing the reintroduction of experimental populations, was added to the Act in 1982. See 16 U.S.C. 1539(j); Pub. L. No. 97-304, § 6, 96 Stat. 1411, 1423 (1982). Under section 10(j), the Service may reintroduce an experimental population of a threatened or endangered species in order to further the statute’s conservation goals. 16 U.S.C. § 1539(j). Each reintroduced population must be designated as essential or nonessential, according to whether the population is necessary “to the continued existence” of the species. Id. § 1539(j)(3); 50 C.F.R. § 17.81(c)(2) (1984). While section 10(j) provides the Service with flexibility in its management of experimental populations, such populations must still be managed to “further the conservation of [the] species.” 16 U.S.C. §§ 1533(d), 1539(j)(2)(A); 50 C.F.R. § 17.81(b).
Congress intended for section 10(j) “to encourage the establishment of new or experimental populations of endangered species by providing the Secretary more flexibility in issuing regulations for their protection.” 128 Cong. Rec. 13,183 (June 9, 1982) (emphasis added). In doing so, FWS must consider the best available information about the role of the experimental population in the species’ recovery. See 16 U.S.C. § 1539(j)(2)(B). While the Service “has discretion to issue the regulations it deems necessary and advisable, …the regulation shall provide for the conservation of such species.” Defs. of Wildlife v. Tuggle, 607 F. Supp. 2d 1095, 1116–17 (D. Ariz. 2009) (emphasis added); see also Red Wolf Coal. v. U.S. Fish & Wildlife Serv., 346 F. Supp. 3d 802, 814 (E.D.N.C. 2018) (citing Tuggle). FWS must also consider input from the public and other stakeholders in species conservation efforts under section 10(j), as experimental populations may be designated only after a notice-and-comment rulemaking process, 16 U.S.C. § 1539(j)(2)(B), and after compliance with the National Environmental Policy Act, 42 U.S.C. § 4321 et seq. See also 87 Fed. Reg. at 34,627 (“When the Service proposes to establish an experimental population, the proposed action will be subject to the NEPA process at that time.”).
B. Experimental Populations in the Southeast
Reintroduced populations have been key to reestablishing healthy populations of imperiled wildlife across the country, including well-known reintroductions of iconic wildlife like wolves, California condors, and black-footed ferrets, as well as many lesser-known aquatic species. In addition to furthering conservation of the reintroduced species themselves, such reintroductions can contribute important ecosystem services that benefit humans and the entire ecosystem. Important ecological functions lost to extinction and diminished biodiversity can be replaced by reintroducing ecologically similar species.18Ian D. Lunt et al., Using assisted colonisation to conserve biodiversity and restore ecosystem function under climate change, BIOLOGICAL CONSERVATION (Jan. 2013); Christine J. Griffiths et al., The use of extant non- indigenous tortoises as a restoration tool to replace extinct ecosystem engineers. RESTORATION ECOLOGY (Jan. 11, 2010).
In the Southeast, experimental populations have been authorized for 32 species, representing more than half the total number authorized across the country.1950 C.F.R. § 17.84–85 (1984). These include two terrestrial species—the red wolf and whooping crane—and 30 freshwater aquatic species (seven fishes and 23 invertebrates). See Table 1.
Table 1. List of Experimental Populations Authorized in the Southeast.20Note that not all 10(j) regulations authorizing experimental populations have resulted in successful reintroduction, for various reasons, such as: unsuccessful captive propagation, unsuitable habitat for reintroduction, or unknown reintroduction techniques. See Hunter Sapienza & Ya-Wei Li, Reintroduction: An Assessment of Endangered Species Act Experimental Populations, ENV’T POLICY INNOVATION CTR. (June 2021), https://www.policyinnovation.org/s/EPIC-Experimental-Population-Analysis.pdf.
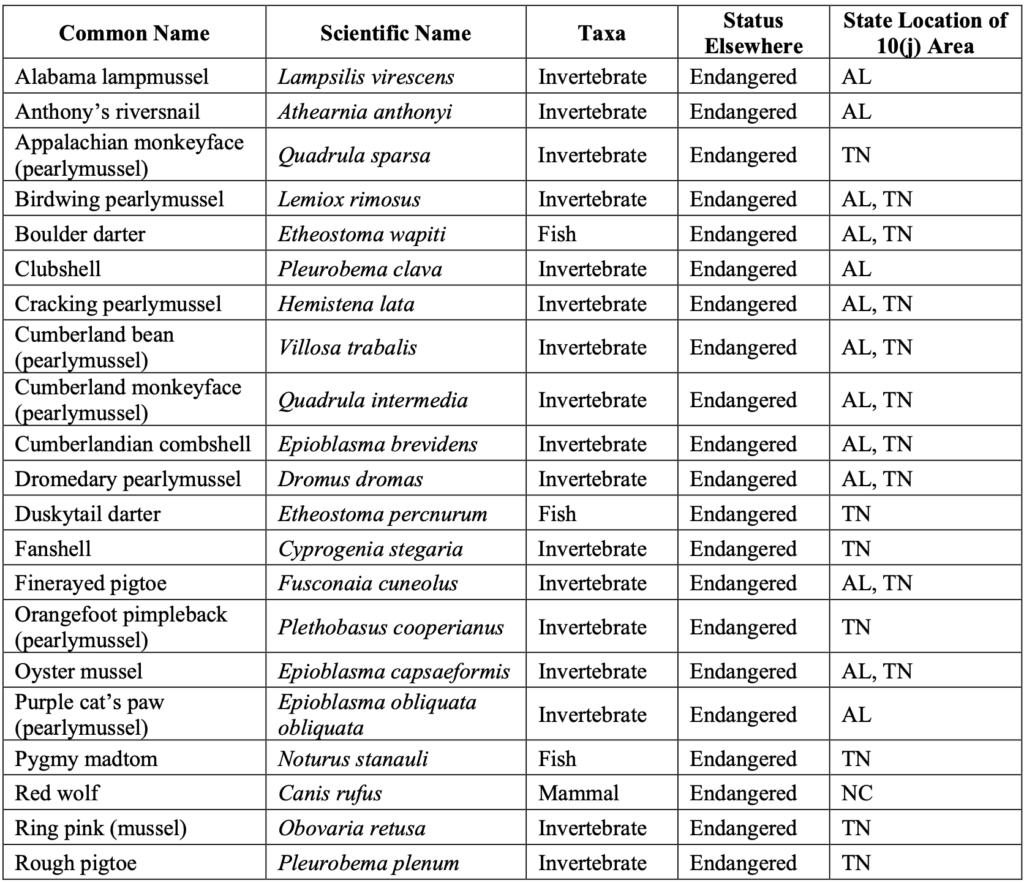
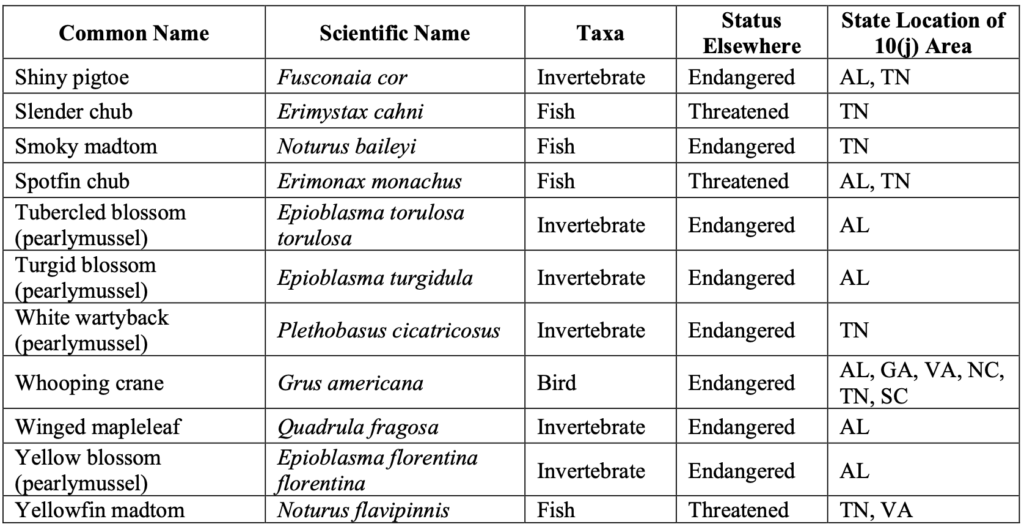
This abundance of aquatic experimental populations reflects the Southeast’s rich freshwater biodiversity. The Southeast is home to some of the most biologically diverse river systems in the United States, serving as hotspots for rare and imperiled species—many of which are endemic to their drainages.21Richard J. Neves et al., Status of aquatic mollusks in the southeastern United States: A downward spiral of diversity, in AQUATIC FAUNA IN PERIL: THE SOUTHEASTERN PERSPECTIVE, 43–85 (George W. Benz & David E. Collins eds., 1997). Freshwater mussels in particular have faced significant declines in the last century and are considered one of the most imperiled taxa in North America.22Wendell R. Haag & James D. Williams, Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels, FRESHWATER BIVALVES (Apr. 28, 2013), provided as Attachment 10. Reintroduction programs for imperiled southeastern aquatic species have been carried out for decades with varying success. For example, the endangered Anthony’s riversnail has been successfully reintroduced into the Wilson Dam tailwater on the Tennessee River and is showing signs of natural reproduction.23U.S. FISH & WILDLIFE SERVICE, ANTHONY’S RIVERSNAIL (ATHEARNIA ANTHONYI) 5-YEAR REVIEW: SUMMARY AND EVALUATION (Mar. 8, 2018). Similarly, experimental populations of four fishes—the smoky madtom, yellowfin madtom, spotfin chub, and duskytail darter—have been reintroduced and are either reestablished or well on their way to becoming reestablished in multiple formerly occupied streams in the Little Tennessee River near Great Smoky Mountains National Park.24J.R. Shute et al., Reintroduction of Four Imperiled Fishes in Abrams Creek, Tennessee, SOUTHEASTERN NATURALIST (2005), provided as Attachment 11; P.L. Rakes et al., Captive propagation and population monitoring of rare southeastern fishes in Tennessee: 2019 (Final Report to Tennessee Wildlife Resources Agency), CHEROKEE NAT’L FOREST, TENN. VALLEY AUTH., & USWFS (2020).
Reintroductions of extirpated freshwater species into suitable habitats—either historic or new—will continue to be crucial for recovering these species and mitigating losses from current and emerging threats of habitat loss and climate change.
The same is true for terrestrial experimental populations such as whooping cranes. In 2000, FWS began efforts to reintroduce an experimental population of whooping cranes into a 20-state area along the eastern United States.25Elizabeth H. Smith, Species Review: Whooping Crane (Grus americana), IUCN CRANE SPECIALIST GROUP (2019), https://savingcranes.org/wp-content/uploads/2022/05/crane conservation strategy whooping crane.pdf. The reintroduction has grown from seven released whooping cranes in 2001 to around 81 as of the end of 2021.26 Infographic, All the Whooping Cranes in the World, INT’L CRANE FOUND. (2021), available at https://savingcranes.org/learn/species-field-guide/whooping-crane/
Because whooping cranes are particularly vulnerable to climate change and may need to shift their range as a result of warming temperatures,27Katherine E. Golden et al., Spatial and temporal predictions of whooping crane (Grus americana) habitat along the US Gulf Coast, CONSERVATION SCI. & PRACTICE (Mar. 30, 2022), provided as Attachment 12. protections for experimental populations could play a pivotal role in this species’ recovery—as well as that of other bird species threatened by climate change.28E.g., Elizabeth Pennisi, Three billion North American birds have vanished since 1970, surveys show, SCI. (Sept. 19, 2019), https://www.science.org/content/article/three-billion-north-american-birds-have-vanished-1970-surveys- show; Kenneth V. Rosenberg et al., Decline of the North American avifauna, SCI. (Oct. 4, 2019), Attachment 13.
III. THE SERVICE’S PROPOSAL WILL FURTHER THE ESA’S PURPOSE IN THE FACE OF ACCELERATING THREATS
The Service’s instant proposal to allow reintroductions outside species’ historic ranges is consistent with the conservation goals of the ESA and necessary to help ecosystems adapt to accelerating threats to species and their habitat—particularly from habitat loss and climate change. As climate change continues to alter ecosystems, threatened and endangered species may increasingly benefit from populations reintroduced into areas beyond their historic range. This is particularly true in the biodiverse Southeast, where high baseline levels of habitat loss may prevent imperiled species from migrating into new areas on their own. Therefore, the Service should remove the “probable historic range” limitation from its regulations, which is consistent with the intent and purposes of section 10(j). FWS should also finalize minor clarifying changes that do not alter the scope of the regulations, but it should reconsider its more substantial change to redefine suitable habitat as “necessary to support one or more life stages,” as explained in greater detail below.
A. Removing the “Probable Historic Range” is Supported by the Statute
Section 10(j) was designed “to provide a vehicle for the development of special regulations for each experimental population that will address the particular needs of that population.” H.R. Rep. No. 97-835, 128 Cong. Rec. at 24,158 (Sept. 17, 1982) (emphases added). In other words, Congress intended to provide FWS with the authority to make thorough, case-by-base scientific determinations about experimental populations—including where to place them. Before establishing an experimental population, FWS must “determine[] that such release will further the conservation of such species.” 16 U.S.C. § 1539(j)(2)(A). The only further statutory limitation on the geography of experimental populations is that the experimental and natural populations of the species be “wholly separate.” Id. § 1539(j)(1). The ESA therefore empowers FWS to determine whether and where releasing an experimental population “will further conservation of such species.” Id. § 1539(j)(2)(A).
When FWS first issued regulations to implement section 10(j) in 1984, the agency explained that it regarded looking to a species’ historic range as the “most biologically acceptable approach” for determining a suitable habitat for experimental populations. 46 Fed. Reg. 33,885, 33,890 (Aug. 27, 1984). The agency explained that the “Service must commit itself to ecosystem protection and to programs for the conservation of listed species,” reasoning that still supports the Service’s instant revisions. Id. The Service also previously expressed concerns about releasing species beyond their historic range based on an assumption that such areas would represent unsuitable habitat or would subject the reintroduced population to “doubtful survival chances”—ideas that no longer apply to a human-altered and climate-changed landscape where such reintroductions may actually be necessary to support the goals of the ESA. See id. Even then, the agency’s own 1984 regulations envisioned a need to authorize reintroductions beyond species’ probable historic range in the “extreme case” if that habitat had become unsuitable. 50 C.F.R. § 70.81(b) (1984).
B. The Proposed Rule Will Enhance FWS’s Ability to Manage Species Adaptation in Response to Climate Change
Unfortunately, a lack of suitable habitat has become less an “extreme case” and more an accelerating global norm in the years since FWS first promulgated regulations under section 10(j). As the Service acknowledges in its preamble to the Proposed Rule, “it did not anticipate the impact of climate change on species and their habitats,” which it has “since learned … is causing, or is anticipated to cause, many species’ suitable habitat to shift outside of their historical range.” 87 Fed. Reg. at 34,625. Indeed, in the 40 years since section 10(j) was adopted, climate change and habitat loss have altered and will continue to alter species’ distribution in significant ways, meaning historic range may in some cases no longer represent the most potentially successful area for species reintroduction. As the Service correctly notes, all or part of a species’ historic range may cease to be suitable for that species, while new areas may begin to provide more of the conditions necessary for the same species to thrive. See id.29The Service explains that its proposed rule would allow reintroductions outside of a species’ historic range “under appropriate circumstances,” including “instances where little to no habitat remains within the historical range of a species or where formerly suitable habitat within the historical range has undergone, or is undergoing, irreversible decline or change, rendering it unable to support one or more life history stages for the species.” 87 Fed. Reg. at 34,625.
The acceleration of climate change-induced habitat shifts, compounded by habitat destruction, has introduced a new urgency to such adaptation and reintroduction efforts. Species adapt as quickly as possible to their changing environments, but the rate of current change is so rapid—and the movement of species so hampered by existing habitat fragmentation—that intervention in the form of species reintroduction into new, previously uninhabited areas may become increasingly necessary to facilitate the migration of species ranges and ultimately prevent species extinction.30See Intergovernmental Science-Policy Platform on Biodiversity & Ecosystem Services, Summary for Policymakers, in GLOBAL ASSESSMENT REPORT ON BIODIVERSITY AND ECOSYSTEM SERVICES (S. Díaz et al. eds., 2019), available at https://ipbes.net/global-assessment, at 12 (“The widespread declines in geographic distribution and population sizes of many species make clear that, although evolutionary adaptation to human caused drivers can be rapid, it has often not been sufficient to mitigate them fully.”). For example, the IPCC notes that “most…freshwater molluscs will not be able to keep up [their geographical range shifts] at the rates projected under RCP4.5 and above in flat landscapes in this century.”31IPCC, Summary for Policymakers, in CLIMATE CHANGE 2014: AR5 SYNTHESIS REPORT (R.K. Pachauri & L.A. Meyer eds., 2014), https://www.ipcc.ch/site/assets/uploads/2018/02/AR5 SYR FINAL SPM.pdf, at 13. Their fate is further compounded by habitat blockages (e.g., dams) that prevent larval dispersion by host fish into previously native habitats. In the biodiverse rivers of the Southeast, increased flexibility to establish experimental populations in new areas will be imperative to give freshwater invertebrates a fighting chance against climate change. Indeed, the scientific literature describes methods to guide managed relocation and/or introduction of experimental populations as species’ habitats are altered by changes in climate and ranges shift.32Aviv Karasov-Olson et al., Co-development of a risk assessment strategy for managed relocation, ECOLOGICAL SOLUTIONS & EVIDENCE (Aug. 9, 2021), provided as Attachment 14. Thus, allowing for reintroductions beyond a species’ historic range will further the “conservation and survival of … species” as the ESA requires. 16 U.S.C. § 1533(f).
In addition to climate change and habitat loss concerns, the use of “historic range” as a proxy for suitable habitat or species occurrence has become increasingly unreliable. Since the 1984 regulations were adopted, studies have shown that historical range is not always an appropriate guide for selecting the best areas for species reintroductions. For example, species distribution maps, which are used to describe historical range, reflect human knowledge of species’ occurrence, meaning a species may be deemed absent from a location due to lack of detection or recording.33Jennifer K. Frey, Inferring species distributions in the absence of occurrence records: An example considering wolverine (Gulo gulo) and Canada lynx (Lynx canadensis) in New Mexico, BIOLOGICAL CONSERVATION (June 2006). Furthermore, human error or flawed methodology in sampling or identifying species may produce an inaccurate or incomplete record of species occurrences.34Rex Dalton, Ornithologists stunned by bird collector’s deceit, NATURE (Sept. 15, 2005); Ant Maddock & Morné A. du Plessis, Can species data only be appropriately used to conserve biodiversity?, BIODIVERSITY & CONSERVATION (May 1999). Accordingly, the removal of “probable historic range” as a criterion for experimental population siting will allow the Service to be guided by other relevant evidence that may maximize the recovery chances of experimental populations.
C. The Service’s Clarifying Changes Must Also Align with the ESA
The Service also proposes to make several “minor changes to clarify the existing regulations.” 87 Fed. Reg. at 34,625. FWS explains that these changes “are not intended to alter the substance or scope of the regulations.” Id. We support most of these changes and agree that they add clarity to the rules and in turn better support implementation of section 10(j).35The Service’s proposed new rule text also replaces “shall” with “will” in a few provisions, including the current requirement at 50 C.F.R. § 17.81(b) that the Service “shall utilize the best scientific and commercial data available” in determining such release will further the conservation of the species (emphasis added). The Service does not address these changes in the preamble to its proposed rule. To the extent the Service intends for this to reflect a policy choice about whether the best available science must be used in such determinations, it goes beyond a “minor change[] to clarify the existing regulations.” 87 Fed. Reg. at 34,625. The Service should simply retain the current “shall” language or else properly notify the public of this change. Specifically, we support introducing the phrase “species-specific rules,” removing the word “natural” in reference to habitat, adding Tribal governments to the list of entities the Service will consult in issuing such rules, and replacing the undefined term “natural” with “nonexperimental.” See id. at 34,626.
The Service’s proposal to replace “suitable natural habitat” with “habitat that is necessary to support one or more life history stage,” however, goes beyond a clarifying change and may unintentionally limit the Service’s ability to reintroduce experimental populations. Indeed, the Service recently repealed a definition of “habitat” with similarly limiting language on the grounds that defining habitat was unnecessary and in tension with the conservation purposes of the ESA. See Regulations for Listing Endangered and Threatened Species and Designating Critical Habitat, 87 Fed. Reg. 37,757, 37,757 (June 24, 2022) (repealing definition of habitat that included the phrase “necessary to support one or more life processes”). The Service should likewise refrain from unintentionally restricting what it may consider appropriate habitat for an experimental population under section 10(j).
The phrase “necessary to support” could impose a higher standard than the Service has previously required in assessing possible habitat areas that would otherwise suffice to support a reintroduced population or provide a benefit to the species—for example, degraded or edge habitat used by the species, or areas of habitat that may not currently be “necessary” to support the species but may in the future prove necessary as habitats shift in response to climate change. Similarly, some species’ life history and habitat needs may be poorly understood, rendering a finding that habitat is “necessary to support one or more life history stage” difficult. See 87 Fed. Reg. 37,759 (noting confusing application of “necessary to support” terminology that could be “affected by how much is known about a given species”); id. at 37,768 (noting that “differing and potentially conflicting interpretations could arise regarding” meaning of “necessary to support”). Other provisions in the regulations already require the Service to consider a proposed experimental population’s chances of survival and contributions to recovery of the species, which will necessarily call upon the Service to consider whether an area includes habitat that will support such outcomes. See 50 C.F.R. 17.81(b)(2), (3). The Service should retain the current regulation’s reference to “suitable … habitat”36As noted above, we support the removal of the word “natural” from the current paragraph (a) at 50 C.F.R. § 17.81. or simply refer to “habitat” without qualification.
IV . CONCLUSION
As explained above, we applaud the Service’s forward-thinking proposal to look outside species’ historic ranges when considering the best places to establish experimental populations. Such a change will enable the Service to better fulfill its duties to conserve and recover species in the face of accelerating threats, including those from climate change. As the Service works to finalize this change, it should carefully consider whether its other “clarifying” changes may have unintended consequences, including regarding its proposed replacement of “suitable habitat.” We look forward to continuing to work with the Service to conserve and recover imperiled species in the Southeast.
Sincerely,
Ramona H. McGee
Senior Attorney and Wildlife Program Leader
Southern Environmental Law Center
Henry Gargan
Associate Attorney
Southern Environmental Law Center
Melissa L. Edmonds
Science & Policy Analyst
Southern Environmental Law Center
On behalf of:
Animal Welfare Institute
Johanna Hamburger
Terrestrial Wildlife Program Director and Senior Staff Attorney
Carolina Wetlands Association
Rick Savage
Executive Director
Center for Biological Diversity
Noah Greenwald
Endangered Species Director
Chattooga Conservancy
Nicole Hayler
Director
Cherokee Forest Voices
Catherine Murray
Director
Coalition to Protect America’s National Parks
Mike Murray
Chair
Coastal Plain Conservation Group
Andy Wood
Director
Coosa Riverkeeper
Justinn Overton
Staff Riverkeeper & Executive Director
Defenders of Wildlife
Ben Prater
Director, Southeast Program
Endangered Species Coalition
Leda Huta
Executive Director
Friends of Buckingham
Chad Oba
President
Friends of Nelson
Mary Eiserman
President
Glynn Environmental Coalition
Rachael Thompson
Executive Director
Highlanders for Responsible Development
Rick Lambert
President
MountainTrue
Bob Gale
Ecologist & Public Lands Director
National Parks Conservation Association
Rachel Kenigsberg
Associate General Counsel
North Carolina League of Conservation Voters
Carrie Clark
Executive Director
North Carolina Wildlife Federation
Tim Gestwicki
CEO
Shoals Environmental Alliance
Charles L. Rose
President
Sierra Club
Karimah Schoenhut
Staff Attorney
Sierra Club, Tennessee Chapter
Axel C. Ringe
Water Quality Chair
South Carolina Wildlife Federation
Sara K. Green
Executive Director
Tennessee Riverkeeper
David Whiteside
Executive Director
The Clinch Coalition
Sharon Fisher
President
Upstate Forever
Scott Park
Glenn Hilliard Director of Land Conservation
Virginia Conservation Network
Patrick L. Calvert
Senior Policy & Campaign Manager
Virginia Wilderness Committee
Mark Miller
Executive Director
